PEP-010
PEP-Therapy and Institut Curie are conducting a first-in-human Phase Ia/b clinical trial of PEP-010 for the treatment of advanced solid tumors and in particular:
- Platinum-Resistant Ovarian Cancer (PROC)
- Pancreatic ductal adenocarcinoma (PDAC).
The clinical trial (NCT 04733027) is a dose escalation trial with expansion cohorts. The study is also conducted at three other clinical oncology centers, Gustave Roussy, the François Baclesse Center and Institut de Cancérologie de l’Ouest.
The trial is led by Professor Christophe Le Tourneau, Medical Oncologist at Institut Curie and Head of the Department of Drug Development and Innovation (D3i), and Principal Investigator of the trial. Named ‘CleverPeptide’, it is an open-label, non-controlled, multicenter clinical trial.
Our lead product, PEP-010, has completed its Phase Ia clinical study. Results obtained in 34 patients with recurrent and/or metastatic solid tumors, showed:
- Favorable safety and tolerability profiles
- Recommended Phase 2 Dose (RP2D) determined in monotherapy and in combination with paclitaxel
- Encouraging preliminary signals of antitumor activity:
- 13 patients have shown Partial Response or Stable Disease,
- 2 confirmed Partial Responses in combination with paclitaxel in ovarian and pancreatic cancers.
The Phase Ib clinical study is underway, focusing on two indications, PROC and PDAC, to confirm the safety and further investigate preliminary antitumor signals observed in Phase Ia. A new combination of PEP-010 with a standard-of-care in these two indications, gemcitabine, will also be evaluated.
The U.S. Food and Drug Administration (FDA) has granted Orphan Drug designation (ODD) to PEP-010 for the treatment of pancreatic cancer.
PEP-010 is a first-in-class therapeutic peptide.
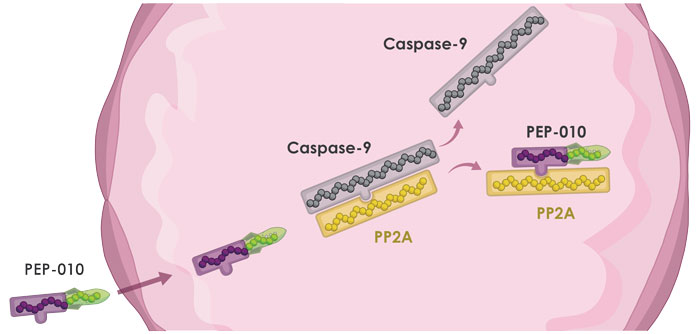
PEP-010, PEP-Therapy’s lead product, dissociates the interaction between Caspase-9 and PP2A, two key proteins in the apoptotic pathway. PP2A and Caspase-9, when released, restore normal apoptosis (physiological cell death) in cancer cells.
PEP-010 comprises two features in a single peptide, based on our innovative and patented CP&IP (Cell Penetrating & Interfering Peptides) technology made of:
An optimized Cell-Penetrating Peptide (CPP), as a “shuttle” to deliver the active peptide into the cellular cytosol;
A short Interfering Peptide, specifically designed to disrupt a Protein-Protein Interaction leading to the inhibition of key pathological mechanisms without altering physiological mechanisms. In the case of PEP-010, the interfering peptide disrupts the interaction between Caspase-9 and PP2A.
PEP-010 has shown a good safety profile and anti-tumor activity in several preclinical models with tumor growth inhibition up to 85% in breast and ovary Patient Derived Xenograft (PDX) tumor models. Additive effect of PEP-010 in combination with chemotherapies were also observed. Publications are available upon request, please contact us.
PIPELINE
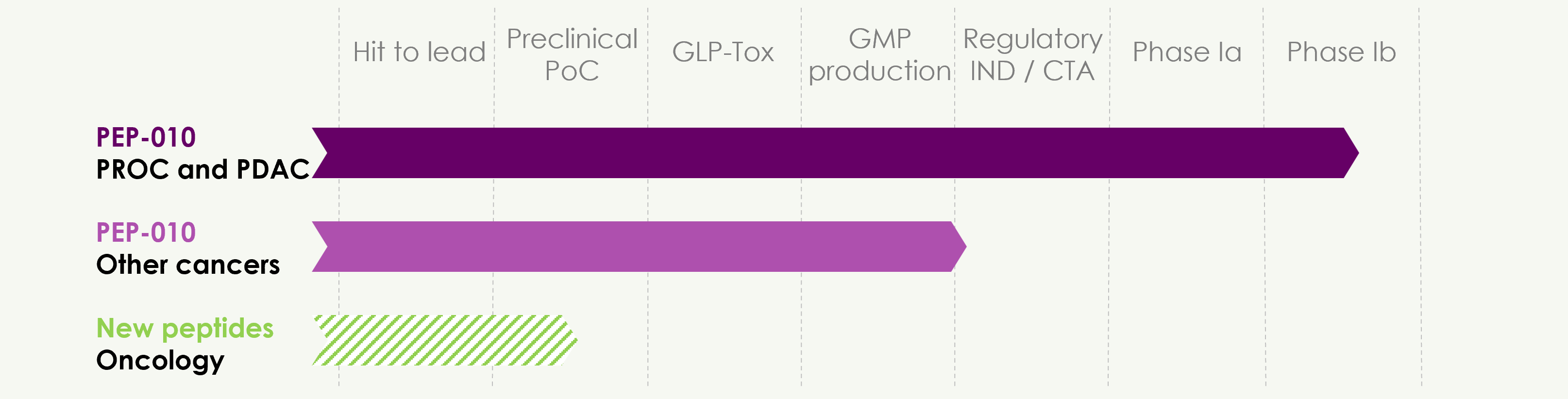
Based on the proof-of-concept and the experience gained on its lead product, PEP-010, PEP-Therapy develops a pipeline of peptide-based products in oncology.
Main publications
Publications
1. Lacroix et al., 2025. The pro-apoptotic peptide PEP-010 triggers apoptosis in pancreatic tumor models in monotherapy and in combination with gemcitabine independently of the KRAS status. Cancer Lett. 10;630:217896 doi: 10.1016/j.canlet.2025.217896
2. Germini D et al., 2024. A translational study for biomarker identification of PEP-010, a pro-apoptotic peptide restoring apoptosis in cancer models. Biochim Biophys Acta Mol Basis Dis. 30;1871(1):167492. doi: 10.1016/j.bbadis.2024.167492
3. Lacroix A et al., 2024. The first-in-class pro-apoptotic peptide PEP-010 is effective in monotherapy and in combination with paclitaxel on resistant ovarian adenocarcinoma cell models. Front Pharmacol. 7;15:1444973. doi: 10.3389/fphar.2024.1444973.
4. Dorgham K et al., 2022. Binding and Kinetic Analysis of Human Protein Phosphatase PP2A Interactions with Caspase 9 Protein and the Interfering Peptide C9h. Pharmaceutics. 27;14(10):2055. doi: 10.3390/pharmaceutics14102055.
5. Bruzzoni-Giovanelli H et al., 2018. Interfering peptides targeting protein-protein interactions: the next generation of drugs? Drug Discov Today. 23(2):272-285. doi: 10.1016/j.drudis.2017.10.016
6. Zhang X et al., 2017. Identification and characterization of novel enhanced cell penetrating peptides for anti-cancer cargo delivery. Oncotarget. 11;9(5):5944-5957. doi: 10.18632/oncotarget.23179
7. Fominaya J et al., 2015. Enhanced serum proteolysis resistance of cell-penetrating peptides. Ther Deliv. 6(2):139-47. doi: 10.4155/tde.14.100.
8. Arrouss I et al., 2015. Cell penetrating peptides as a therapeutic strategy in chronic lymphocytic leukemia. Protein Pept Lett. 22(6):539-46. doi: 10.2174/0929866522666150216115352.
9. Arrouss I et al., 2013. Specific targeting of caspase-9/PP2A interaction as potential new anti-cancer therapy. PLoS One. 23; 8(4):e60816. doi: 10.1371/journal.pone.0060816.
10. Janssens V, Rebollo A. 2012. The role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cells. Curr Mol Med. 12(3):268-87. doi: 10.2174/156652412799218930.
Proceedings in international meetings
1. Lacroix A et al., 2024. PEP-010, a first-in-class clinical-stage proapoptotic peptide is efficient in vitro and in ovo on pancreatic cancer in monotherapy and in combination with different chemotherapies. Abstract, EACR annual meeting: Innovative Cancer Science; Rotterdam, Netherlands. Mol Oncol 18 (Supplement 1): 0302. https://doi.org/10.1002/1878-0261.13682
2. Lacroix A et al., 2024. PP2A triggers the cell death signaling cascade after treatment with PEP-010, a first-in-class clinical-stage pro-apoptotic peptide. Abstract, EACR annual meeting: Innovative Cancer Science; Rotterdam, Netherlands. Mol Oncol 18 (Supplement 1): 0252. https://doi.org/10.1002/1878-0261.13682
3. Du Rusquec P., et al 2023. A first-in-human Phase 1a/b of PEP-010, a proapoptotic bifunctional peptide, administered as single agent and in combination with paclitaxel in patients with recurrent and/or metastatic solid cancer: results from the dose escalation study. Abstract, AACR-NCI-EORTC international conference: molecular targets and cancer therapy; Boston, MA. Mol Cancer Ther 22 (12-Supplement):B046. https://doi.org/10.1158/1535-7163.TARG-23-B046
4. Farhat et al., 2023. The proapoptotic peptide PEP-010 is efficient on several models of different tumor origins and it can be monitored by pharmacodynamic biomarker candidates in clinical practice. Abstract, AACR Annnual Meeting; Orlando, FL. Cancer Res 83 (7_Supplement): 6129. https://doi.org/10.1158/1538-7445.AM2023-6129
5. Lebel-Binay et al., 2018. PEP-010, a cell penetrating & interfering peptide as a new therapeutic approach in breast cancer. Abstract, AACR Annual meeting; Chicago, IL. Cancer Res 78 (13_Supplement): 3904. https://doi.org/10.1158/1538-7445.AM2018-3904
6. Nemati F et al., 2011. Targeting caspase-9 PP2A interaction as a new antitumor strategy. Abstract, AACR-NCI-EORTC international conference: molecular targets and cancer therapy; San Francisco, CA. Mol Cancer Ther 10 (11_Supplement): A205. https://doi.org/10.1158/1535-7163.TARG-11-A205